Clopidogrel (Plavix) – CYP2C19
Rationale
This drug gene interaction (DGI) pertains to the interaction between the cytochrome P450 2C19 (CYP2C19) gene and clopidogrel. Clopidogrel (brand name Plavix®) is used to prevent heart attack and stroke in persons who have recently had a heart attack, stroke, or blood circulation disease (peripheral vascular disease). Clopidogrel works by preventing specific blood cells called platelets from sticking together and forming harmful clots, thus clopidogrel is an ‘antiplatelet drug’ and helps keep blood flowing smoothly in the body. It is also used with aspirin to treat new or worsening chest pain and to prevent blood clots after certain procedures such as a cardiac stent.
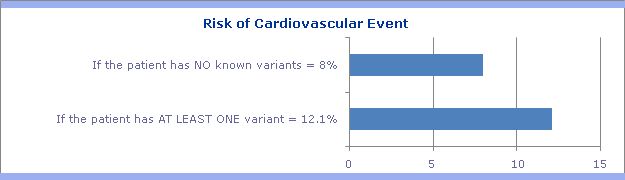
Extensive literature and FDA warning labels indicate patients with genetically reduced CYP2C19 function demonstrate lower systemic exposure to the active metabolite of clopidogrel, diminished anti-platelet responses, and generally exhibit higher cardiovascular event rates following a coronary stent procedure than do patients with normal CYP2C19 function.
Information presented on this page is based on evidence provided by the Clinical Pharmacogenomics Implementation Consortium (CPIC®). CPIC provides peer-reviewed, updated, evidence-based, and freely accessible guidelines for implementing pharmacogenomic results into actionable prescribing decisions for providers. CPIC guidelines include standardized terminology and a systematic grading of evidence and clinical recommendations published in a leading journal (Clinical Pharmacology and Therapeutics).
Genetic Variant Information
The CYP2C19 (sounds like “sip-2-see-19”) gene encodes the CYP2C19 enzyme, which is a member of the cytochrome P450 enzyme family. There are different CYP2C19 gene versions, or variants, that affect how well clopidogrel is metabolized. Some genetic variants result in a non-functioning or decreased functioning CYP2C19 enzyme, while other variants result in a normal functioning CYP2C19 enzyme. In some cases, multiple copies of functioning variants can lead to increased CYP2C19 activity. Everyone has two copies of the CYP2C19 gene, known as alleles. Based on both alleles, individuals are classified into different ‘metabolizer statuses’ (phenotype) depending on their genetic information (genotype).
See chart below for a description of each metabolizer status and any implications for treatment.
CYP2C19 metabolizer status (phenotype) |
Alleles (genotype) |
Implication for clopidogrel |
|---|---|---|
| Poor metabolizer | TWO no-function alleles | Significantly reduced clopidogrel active metabolite formation; increased on-treatment platelet reactivity; increased risk for adverse cardiac and cerebrovascular events. |
| Intermediate metabolizer | ONE normal function allele AND ONE no-function allele
OR ONE increased function allele AND ONE no-function allele |
Reduced clopidogrel active metabolite formation; increased on-treatment platelet reactivity; increased risk for adverse cardiac and cerebrovascular events. |
| Normal metabolizer | TWO normal function alleles | Normal clopidogrel active metabolite formation; normal on-treatment platelet reactivity. |
| Rapid metabolizer | ONE normal function allele AND ONE increased function allele | Normal or increased clopidogrel active metabolite formation; normal or lower on-treatment platelet reactivity. No association with higher bleeding risk. |
| Ultrarapid metabolizer | TWO increased function alleles | Normal or increased clopidogrel active metabolite formation; normal or lower on-treatment platelet reactivity. No association with higher bleeding risk. |
Patient’s genetic information is reported as a diplotype and this corresponds to a phenotype (i.e. metabolizer status). To see an interpretation table assigning metabolizer status by genetic variant, click here to visit CPIC and scroll down to click and download “CYP2C19 diplotype-phenotype table”.
Results Interpretation
Clopidogrel is a medicine used to manage several different conditions and your genes can affect how well the drug works. CPIC updates guidelines on how to best use these genetic results to support patient care. To view dosing recommendations for clopidogrel based on CYP2C19 phenotypes, click on the most recent guideline publication on CPIC’s website and scroll down to Table 2.
Please review the FDA packet insert for additional clinical considerations such as contradictions as well as dose adjustments based on age, organ function, and drug-drug interactions.
Quality of Results
Genotyping for CYP2C19 was performed within a certified DNA laboratory at Vanderbilt University Medical Center that is in full compliance with all guidelines established by the government as regulated by the Centers for Medicare & Medicaid Services under the Clinical Laboratory Improvement Act of 1988. This validated clinical laboratory developed test is carried out with strict adherence to protocols outlined by the College of American Pathology. The performance of the assay is closely monitored and the accuracy of the results is determined to be > 99%.
Supporting Evidence
This link will take you to the main page on the CPIC website relating to CYP2C19 and clopidogrel. On the site, you will find links to the main guideline publication and all supplementary information including a table that reports variant frequencies across different races/ethnic groups, a table that defines genetic variants, and a table that provides a phenotype interpretation (i.e. metabolizer status). Additionally, examples of point of care clinical decision support can be found at the bottom of the page.
Please note that additional drugs may have CYP2C19 interactions. For more information on drug-gene interactions, please use the search feature on the CPIC website.
Additionally, a comprehensive evidence summary has been provided by the VUMC Informatics Center Knowledge Management (KM) Team. This information was developed by the KM team in response to questions from VUMC clinicians and researchers about topics related to genetics and health. The syntheses provided are copyrighted and should not be re-used without permission.
